
Ikbel Hadj Hassine 1, Manel Ben M’hadheb 1, Luis Menéndez-Arias 2
Affiliations Expand
- PMID: 35458571
- PMCID: PMC9024455
- DOI: 10.3390/v14040841
Abstract
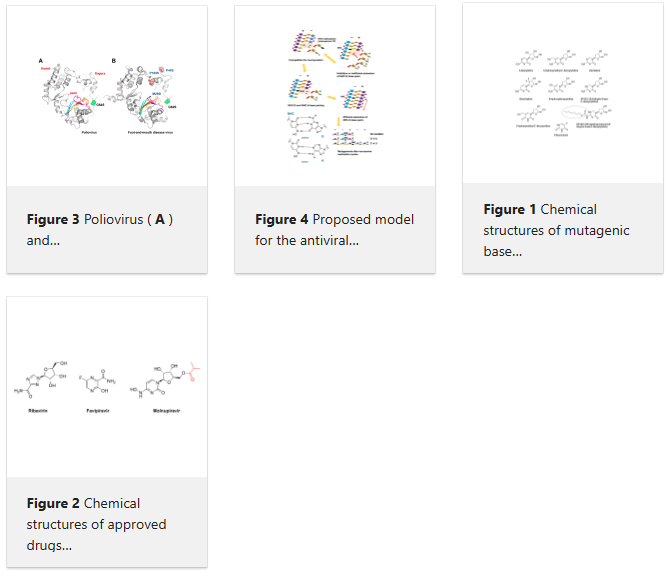
In RNA viruses, a small increase in their mutation rates can be sufficient to exceed their threshold of viability. Lethal mutagenesis is a therapeutic strategy based on the use of mutagens, driving viral populations to extinction. Extinction catastrophe can be experimentally induced by promutagenic nucleosides in cell culture models. The loss of HIV infectivity has been observed after passage in 5-hydroxydeoxycytidine or 5,6-dihydro-5-aza-2′-deoxycytidine while producing a two-fold increase in the viral mutation frequency. Among approved nucleoside analogs, experiments with polioviruses and other RNA viruses suggested that ribavirin can be mutagenic, although its mechanism of action is not clear. Favipiravir and molnupiravir exert an antiviral effect through lethal mutagenesis. Both drugs are broad-spectrum antiviral agents active against RNA viruses. Favipiravir incorporates into viral RNA, affecting the G→A and C→U transition rates. Molnupiravir (a prodrug of β-d-N4-hydroxycytidine) has been recently approved for the treatment of SARS-CoV-2 infection. Its triphosphate derivative can be incorporated into viral RNA and extended by the coronavirus RNA polymerase. Incorrect base pairing and inefficient extension by the polymerase promote mutagenesis by increasing the G→A and C→U transition frequencies. Despite having remarkable antiviral action and resilience to drug resistance, carcinogenic risks and genotoxicity are important concerns limiting their extended use in antiviral therapy.
Keywords: HIV; RNA polymerase; SARS-CoV-2; error catastrophe; favipiravir; lethal mutagenesis; molnupiravir; nucleoside analogs; ribavirin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
- Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: The special case of molnupiravir.Waters MD, Warren S, Hughes C, Lewis P, Zhang F.Environ Mol Mutagen. 2022 Jan;63(1):37-63. doi: 10.1002/em.22471.PMID: 35023215 Review.
- Viral error catastrophe by mutagenic nucleosides.Anderson JP, Daifuku R, Loeb LA.Annu Rev Microbiol. 2004;58:183-205. doi: 10.1146/annurev.micro.58.030603.123649.PMID: 15487935 Review.
- Extinction of Zika Virus and Usutu Virus by Lethal Mutagenesis Reveals Different Patterns of Sensitivity to Three Mutagenic Drugs.Bassi MR, Sempere RN, Meyn P, Polacek C, Arias A.Antimicrob Agents Chemother. 2018 Aug 27;62(9):e00380-18. doi: 10.1128/AAC.00380-18. Print 2018 Sep.PMID: 29914957 Free PMC article.
- Decoding molnupiravir-induced mutagenesis in SARS-CoV-2.Menéndez-Arias L.J Biol Chem. 2021 Jul;297(1):100867. doi: 10.1016/j.jbc.2021.100867. Epub 2021 Jun 9.PMID: 34118236 Free PMC article.
- β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells.Zhou S, Hill CS, Sarkar S, Tse LV, Woodburn BMD, Schinazi RF, Sheahan TP, Baric RS, Heise MT, Swanstrom R.J Infect Dis. 2021 Aug 2;224(3):415-419. doi: 10.1093/infdis/jiab247.PMID: 33961695 Free PMC article.
Cited by
- SARS-CoV-2: pathogenesis, therapeutics, variants, and vaccines.Li X, Mi Z, Liu Z, Rong P.Front Microbiol. 2024 Jun 13;15:1334152. doi: 10.3389/fmicb.2024.1334152. eCollection 2024.PMID: 38939189 Free PMC article. Review.
- Clinico-Virological Outcomes and Mutational Profile of SARS-CoV-2 in Adults Treated with Ribavirin Aerosol for COVID-19 Pneumonia.Morsica G, Messina E, Bagaglio S, Galli L, Lolatto R, Sampaolo M, Barakat M, Israel RJ, Castagna A, Clementi N.Microorganisms. 2024 Jun 5;12(6):1146. doi: 10.3390/microorganisms12061146.PMID: 38930529 Free PMC article.
- Small Molecule Drugs Targeting Viral Polymerases.Palazzotti D, Sguilla M, Manfroni G, Cecchetti V, Astolfi A, Barreca ML.Pharmaceuticals (Basel). 2024 May 20;17(5):661. doi: 10.3390/ph17050661.PMID: 38794231 Free PMC article. Review.
- In-Host HEV Quasispecies Evolution Shows the Limits of Mutagenic Antiviral Treatments.Colomer-Castell S, Gregori J, Garcia-Cehic D, Riveiro-Barciela M, Buti M, Rando-Segura A, Vico-Romero J, Campos C, Ibañez-Lligoña M, Adombi CM, Cortese MF, Tabernero D, Esteban JI, Rodriguez-Frias F, Quer J.Int J Mol Sci. 2023 Dec 6;24(24):17185. doi: 10.3390/ijms242417185.PMID: 38139013 Free PMC article.
- Emerging drug design strategies in anti-influenza drug discovery.Liu C, Hu L, Dong G, Zhang Y, Ferreira da Silva-Júnior E, Liu X, Menéndez-Arias L, Zhan P.Acta Pharm Sin B. 2023 Dec;13(12):4715-4732. doi: 10.1016/j.apsb.2023.08.010. Epub 2023 Aug 14.PMID: 38045039 Free PMC article. Review.
References
- Sanjuán R., Nebot M.R., Chirico N., Mansky L.M., Belshaw R. Viral mutation rates. J. Virol. 2010;84:9733–9748. doi: 10.1128/JVI.00694-10. – DOI – PMC – PubMed
- Domingo E., García-Crespo C., Perales C. Historical perspective on the discovery of the quasispecies concept. Annu. Rev. Virol. 2021;8:51–72. doi: 10.1146/annurev-virology-091919-105900. – DOI – PubMed
- Eigen M. Error catastrophe and antiviral strategy. Proc. Natl. Acad. Sci. USA. 2002;99:13374–13376. doi: 10.1073/pnas.212514799. – DOI – PMC – PubMed
- Eigen M. From Strange Simplicity to Complex Familiarity: A Treatise on Matter, Information, Life and Thought. Oxford University Press; Cary, NC, USA: 2013.
Show all 152 references
